SNPs and Methylation
Prefer to listen to the podcast? Subscribe and listen free:
Early on, I noticed Chloe's MCV trending upward when B12 and folate (folinic acid/5MTHF) supplementation dropped below 1,000 mcg/day.
Through reading and experimentation, I then noticed Chloe's bleeding was abated once thiamine supplementation increased to over 600 mg/day.
On urine organic acid testing, I noticed an elevated 3-hydroxy isovaleric acid, signifying a biotin deficiency, even though she received 1,000 mcg daily. She also had what looked to be seborrheic dermatitis around her mouth that would come and go daily. The rash completely disappeared in a few days with 10,000 mcg of biotin supplementation.
Lastly, I saw a few abnormalities in urine organic acid testing in a few B6-dependent pathways. I started 100 mg of P5P, and within one week, I noticed a dramatic improvement in energy.
Finally, Chloe developed a very subtle case of median rhomboid glossitis. The literature mainly ascribes it to candida. However, it can also occur from a deficiency in B12/folate. This was the straw that broke the camel's back; I knew we missed some genetic abnormality not picked up with whole genome sequencing.
Many clinicians instantly think that supplements are a waste of time and money and that there is no indication beyond the DRI. However, this type of thinking stems from ignorance, and there needs to be more knowledge about cellular biochemistry. I spend every day with our daughter, and I can appreciate the subtle nuances of her day-to-day behavior.
It’s easy to cite the guidelines, but please remember that they are made for the masses and, by definition, can’t be used in personalized medicine—they are quite literally the opposite.
In any event, I knew there was more to the story, so I decided to run additional tests. One of the tests looked at various single-nucleotide polymorphisms (SNPs). A SNP is a single change in the nucleotide base pair. For example, the DNA may replace cytosine (C) with thymine (T). A SNP may be harmful, beneficial, or insignificant. It’s also important to understand that the same SNP can be harmful in one person but beneficial in another if it’s acting as a compensatory mechanism.
You get a copy from your mother and father. Therefore, you will have two alleles when looking at your results (one from mom and one from dad):
+/+: two mutations (homozygous)
+/-: one mutation (heterozygous)
-/-: no mutation
Mostly, you're worse off if you are homozygous for a mutation. But remember, there are exceptions, and not every mutation is considered harmful in every context.
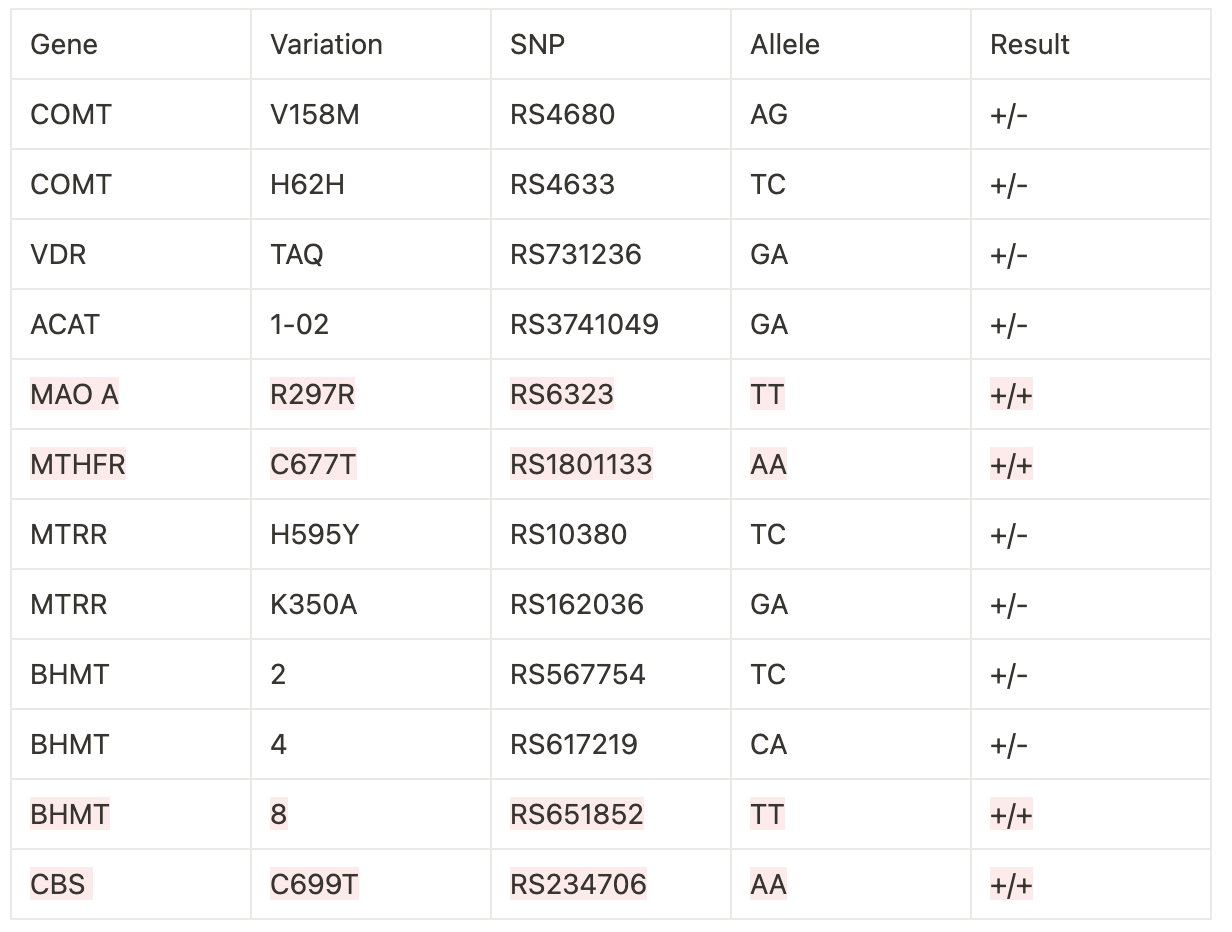
Here are 12 SNPs that I identified that help explain my initial observations:
The SNPs mentioned above play a role in DNA methylation. DNA methylation occurs when S adenosine methionine (SAMe) donates a methyl group (CH3) to the cytosine (c) nucleotide base within our DNA. When a methyl group is donated, this silences or turns “off” gene expression. Conversely, a lack of methylation allows the gene to be transcribed and translated.
Epigenetics is the field that studies how genes are expressed without a change in DNA. Other processes our body uses for gene expression include acetylation, phosphorylation, and ubiquitination. It’s worth mentioning that methylation is also responsible for regulating T cells (preventing autoantibodies), repairing the gut lining, remyelination, neurotransmitter synthesis and transportation, and detoxification of environmental toxins.
“Genetics load the gun, but the environment pulls the trigger.”
- Dr. Judith Stern
Understanding your genetics is crucial to health. Not because it determines a specific disease but because it is the basis for how our environment (nutrition, exercise, sleep, environmental toxins, infection, medications, supplementation, etc.) shapes our future.
The methylation cycle produces other essential compounds, such as glutathione, in addition to the methyl groups. Remember, nothing happens in isolation; everything is interconnected. Everything that enters your body will lead to a chemical reaction (good or bad); nothing results in a neutral effect.
I want to briefly address the significance of each SNP to shed light on how much genetics influences health and disease. I won’t discuss the supplements or the dosages, as I’m constantly adjusting based on behavior and labs. The good news is that everything I’m sharing can be amendable with proper nutrition and supplementation.
This knowledge is truly empowering because there are steps we can take to mitigate disease that would have otherwise appeared for Chloe 5, 10, or 15 years down the road.
COMT
This gene codes for the enzyme Catechol O-methyltransferase, which breaks down and regulates dopamine, norepinephrine, epinephrine, and estrogen. Chloe had very subtle movement disorders that we’ve noticed during times of extreme sleepiness. This is a finding seen in patients who have inborn errors of metabolism, which stem from altered neurotransmitter metabolism/function. She’s had almost a near resolution as we continue to adjust her supplementation.
VDR
This is the vitamin D receptor. Changes at Taq (which she has) may also affect dopamine levels.
ACAT
This stands for acetyl coenzyme A acetyltransferase, which converts acetyl CoA to acetoacetyl CoA. The mutation signifies an increase in this conversion, decreasing acetyl CoA levels. This would then decrease acetylcholine, a neurotransmitter that plays a role in proper muscle function. Because of our daughter’s mitochondrial mutation, coupled with her movement disorders, this is obviously of importance. A decreased muscle tone was one of the earliest signs we noticed that something wasn’t right. She technically hit her gross motor milestones but was always at the outer edge of normal.
Imbalances in acetyl CoA also affect acetylation (which influences SAMe levels), further disrupting gene regulation.
The decrease in acetyl CoA means she’ll have an increase in acetoacetyl CoA, which results in an increased production of ketones. We’ve noted this on every urine organic acids test. Chronically elevated levels of ketones (without a way to use them as energy) can result in weakness, fatigue, and impaired cognition.
ACAT also breaks down the branched-chain amino acids leucine, isoleucine, and valine. If you remember, I noticed elevated levels of 3-hydroxy isovaleric acid (a metabolite from the breakdown of leucine), which is thought to imply a biotin deficiency. However, in her case, it might result from her ACAT mutation.
This mutation is particularly important for us as Chloe also has a mitochondrial mutation. A SNP here also impedes the flow into the citric acid cycle.
Lastly, it’s believed that this SNP will also result in decreased levels of potassium and lithium; lithium is required for proper transport of B12. A decreased lithium will result in reduced utilization of B12 (regardless of what your serum markers show).
MAO-A
This stands for monoamine oxidase A, which is involved in the breakdown of monoamines, such as serotonin, dopamine, epinephrine, norepinephrine, and tyramine. She has reduced MAO-A activity, which can result in an accumulation of neurotransmitters. This can also result in tyramine intolerance, which is described as:
Palpitations
Episodic hypertension
Shortness of breath
Chest pain
Headaches
Flushing
Feelings of anxiety
MTHFR
Methylenetetrahydrofolate reductase (MTHFR) converts 5,10 methylenetetrahydrofolate to 5 methyltetrahydrofolate (5MTHF); this is required to convert homocysteine to methionine. If a patient has this mutation, the conversion to 5MTHF won’t take place, resulting in:
Decreased methyl groups (affecting epigenetics)
A buildup of folate/folic acid (results in an elevated glutamate as the breakdown product). Glutamate results in neuronal excitotoxicity.
Increase in homocysteine, which carries a host of health implications.
After six weeks of tweaking her supplementation, we notice a resolution in her median rhomboid glossitis.
Toxic metals also build up in the body when the methylation pathway isn’t working correctly, which could result in toxicity that otherwise shouldn’t have resulted (we experienced this with aluminum toxicity). Heavy metals can also affect dihydropteridine reductase (DHPR), the enzyme responsible for regenerating tetrahydrobiopterin (BH4). BH4 is a cofactor in synthesizing serotonin, dopamine, and ammonia detoxification. Mutations in CBS (Chloe also has this) also result in elevated ammonia levels. The body will use the limited amount of BH4 to clear ammonia (a priority), leaving less than ideal amounts for serotonin and dopamine (mood and movement).
MTRR
Methionine synthase reductase (MTRR) works with methionine synthase (MTR) to recycle homocysteine back to methionine. MTRR catalyzes the regeneration of methylcobalamin (B12) to be used as a cofactor for MTR.
MTR then uses the methylcobalamin and teams up with 5MTHF. Together, they work to transfer a methyl group from 5MTHF to homocysteine to create methionine. The 5MTHF loses a methyl group, leaving tetrahydrofolate (THF) behind. A mutation in MTRR ultimately results in decreased methylcobalamin (methyl B12) levels.
BHMT
Betaine homocysteine methyltransferase is an enzyme that catalyzes the transfer of a methyl group from trimethylglycine (TMG) to homocysteine to create methionine. After TMG donates its methyl group, it becomes dimethylglycine (DMG).
A mutation here increases the activity of this pathway. Because of the roadblocks seen with B12/5MTHF, an SNP here acts as a compensatory mechanism, allowing methylation to occur. However, it appears that BHMT isn’t found in all tissues. It is mainly found in the liver and kidneys and, to a much smaller extent, in the brain.
CBS
Cystathionine beta synthase converts homocysteine to cystathionine (the first step in the transulfuration pathway). The type of mutation Chloe has increased the conversion of homocysteine to cystathionine.
This is a double-edged sword, as this mutation has positives and negatives.
While it prevents a buildup of homocysteine, it can deplete compounds such as B12. Simultaneously, the transulfuration pathway can create beneficial (glutathione and taurine) or harmful (ammonia and sulfite) end products. However, we can’t control which end products are made, and it is dependent on the various metabolites currently present. Molybdenum helps convert sulfite (harmful) to sulfate, but an increase in sulfite may deplete molybdenum (increasing the requirement).
I hope you can appreciate how interconnected everything is. Our bodies are complex, and there is so much more to health than a CBC, CMP, TSH, and lipid panel. The standard of care is laughable with all the advancements we are making in science.
Unfortunately, it’s quoted that research findings can often take up to 17 years to implement into routine practice. The good news is you don’t have to wait. The answers are out there. Look for them!